Abstract
Graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality in patients receiving allogeneic hematopoietic stem cell transplants (allo-HSCTs). Pre-HSCT conditioning typically consists of irradiation and drug administration which results in the death of rapidly dividing cells and the initiation of a cytokine storm, promoting activation and expansion of donor anti-host alloreactive T cells in HSCT recipients. However, the precise mechanism of cytokine production remains unclear. Cell death following pre-transplant conditioning has promoted the hypothesis that sensors of cytoplasmic DNA damage in GVHD target tissues contribute to cytokine production. One such sensor is Stimulator of Interferon Genes (STING) which following activation induces phosphorylation of IRF3 and IκBα. Although STING activation was recently reported to worsen GVHD after MHC-mismatched allo-HSCT (Fischer J, et al, Sci. Transl. Med. 2017), STING involvement in MHC-matched allo-HSCT has not yet been thoroughly evaluated. Here, using B6-STING knock-out recipients and either MHC-mismatched or matched ("MUD") donor strains we corroborate that STING deficiency worsens - but in contrast - ameliorates GVHD in the former and latter pre-clinical mouse models, respectively.
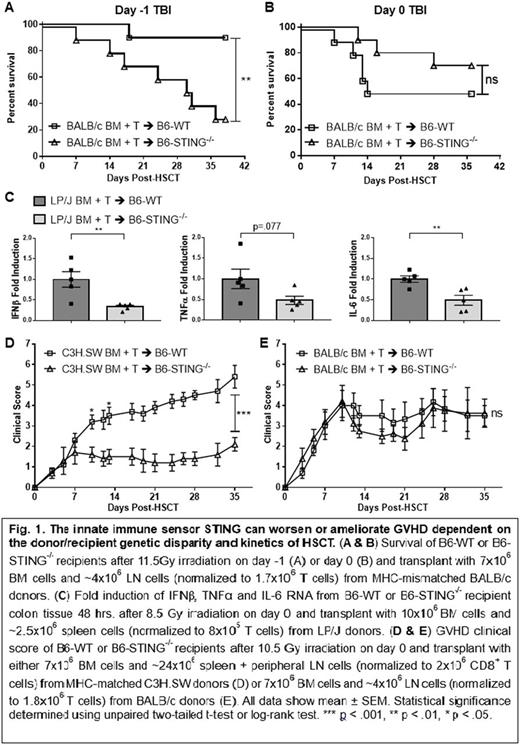
To evaluate the effect of STING deficiency on GVHD after MHC-mismatched allo-HSCT, BALB/c BM + T cells were transplanted into 11.5Gy irradiated B6-WT and B6-STING-/- recipients. Consistent with previously reported results using B6-STINGgt/gt mice, STING-/- recipients experienced increased weight loss and GVHD clinical scores post-HSCT as well as decreased survival relative to WT recipients (Fig. 1A). Notably, when irradiation was given on day 0 instead of day -1 using the same donor/recipient combination no significant difference in GVHD was observed between WT and STING-/- recipients (Fig. 1B & 1E).
We next investigated the role of STING after clinically relevant models of MHC-matched allo-HSCT. To assess for a role by STING immediately after HSCT, we examined cytokine mRNA expression 48 hrs. after transplant of LP/J BM + T cells → 8.5Gy irradiated B6-WT and B6-STING-/- recipients. Colonic tissue from STING-/- recipients had reproducibly >2x reduction in IFNβ, TNFα and IL-6 mRNA compared to WT (Fig. 1C). Importantly, IFNβ is one of the major downstream effector molecules produced as a result of IRF3 phosphorylation, consistent with the notion that STING activation occurs shortly after conditioning and/or allo-HSCT. Notably, LN examination at this time demonstrated fewer donor CD8+ T cells and CD8+CD44hiCD62Llo cells in STING-/- vs WT recipients. In contrast to STING-/- recipients of MHC-mismatched HSCT, MHC-matched STING-/- HSCT recipients experienced decreased weight loss and GVHD clinical scores vs. WT mice. STING-/- recipients contained 20-50% fewer donor lymphocytes and a significantly reduced frequency of activated donor CD4+ and CD8+ T cells in lymphoid tissues on D7. Analysis of peripheral LN and spleen 1-3 mo. post-HSCT revealed a similar profile and a higher frequency of naïve T cells - consistent with their decreased clinical signs of disease. Histological examination of recipient skin also showed higher pathology scores in WT recipients relative to STING-/- recipients. These findings were corroborated using a second MUD model, i.e. C3H.SW → B6 (Fig. 1D). Furthermore, measurement of serum cytokines 6 wks. post-transplant showed that B6-STING-/- recipients had higher levels of the immunosuppressive cytokine IL-10. The effect of donor T cell dose was also examined. Increased donor T cell numbers resulted in enhanced GVHD clinical scores in STING-/- recipients, however, the GVHD remained less severe than that observed in WT. Notably, HSCT studies using fully reconstituted chimeric B6-CD45.1↔B6-STING-/- indicated that STING expression in non-hematopoietic tissues is important for the development of GVHD.
In total, this is the first report we are aware of in which the same pathway appears to differentially impact the outcome of allogeneic HSCT based upon the genetic disparity across the transplant. These findings reveal that STING's contribution to the development of GVHD apparently differs depending on the presence or absence of an MHC-disparity between donors and recipients as well as the timing of the pathway's activation and HSCT.
Levy: Capricor Therapeutics: Consultancy; OccuRx: Research Funding; Shire: Research Funding; Allergan: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal